Sculptra (2x150mg)
The lowest price of the product zł1,220.00 in day 07.01.2026
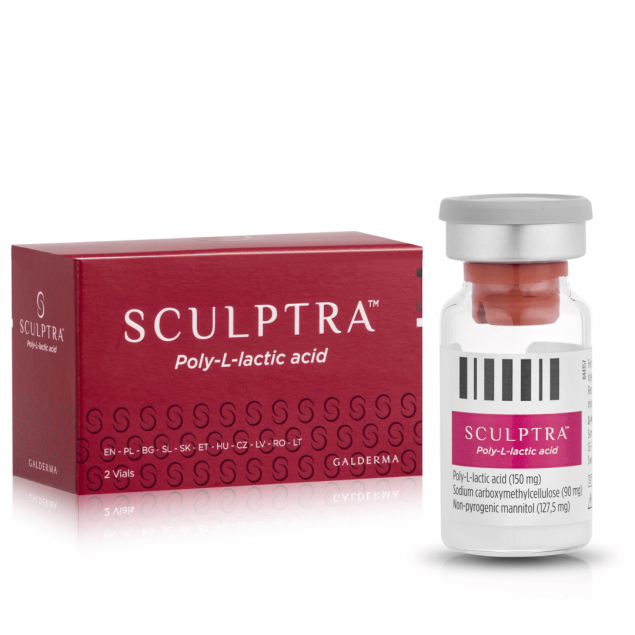
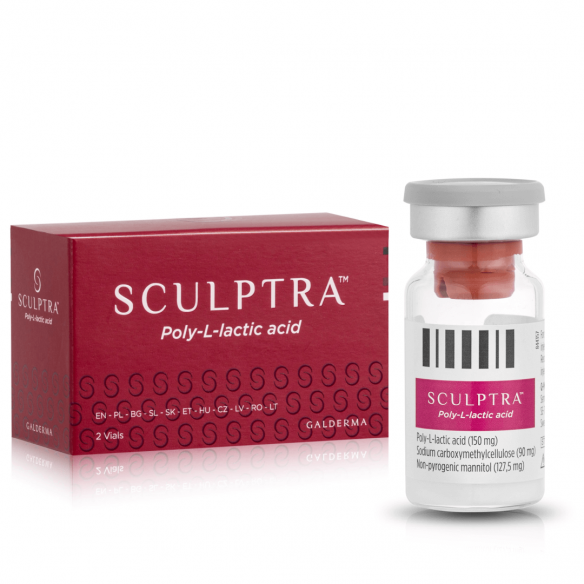
Sculptra is a sterile, non-pyrogenic Class III medical device in the form of a lyophilisate for suspension, containing Poly-L-Lactic Acid (PLLA) microspheres. As a progressive biostimulator, the device induces the synthesis of endogenous collagen by triggering a controlled "foreign body" type immune response within the extracellular matrix. The preparation is used for the physical restoration of tissue volume and the biomechanical correction of deep structural deficits in the dermis and subcutaneous tissue.
mechanism of action
-
induction of neocollagenesis: PLLA microparticles act as a physical scaffold triggering a subclinical inflammatory response, which stimulates fibroblasts to produce collagen fibers, leading to gradual skin densification
-
biomechanical volumetry: the device restores structural tissue integrity by replacing the volume of the preparation with the patient's newly formed connective tissue, resulting in a natural lifting and filling effect
-
gradual tissue integration: the unique shape and size of the PLLA particles allow for uniform integration with surrounding tissues without the risk of sudden migration, ensuring the structural durability of the implant
-
correction of involutional deficits: by stimulating the biosynthesis of structural proteins, the device neutralizes signs of tissue atrophy and facial lipoatrophy
-
hydrodynamic and physicochemical stabilization: the presence of mannitol and carboxymethylcellulose ensures appropriate suspension viscosity and protection against oxidative stress during the peri-procedural phase
indications and intended use
The device is intended for deep implantation by authorized medical personnel to:
-
increase tissue volume (volumetry): correction of skin depressions, ridges, folds, and scars
-
treatment of lipoatrophy: restoration of tissue mass in areas of subcutaneous fat loss (e.g., in HIV patients or due to aging)
-
biomechanical lifting: improvement of tension in lax areas such as the cheeks, jawline (jowls), and décolletage
-
restructuring of large body surfaces: correction of significant soft tissue volume deficiencies
application areas
-
face (nasolabial folds, cheeks, temples, lower facial oval)
-
décolletage
-
other areas requiring significant volumetric correction
composition
-
Poly-L-Lactic Acid (PLLA): 150 mg (active ingredient, collagen stimulator)
-
Sodium carboxymethylcellulose: 90 mg (carrier providing viscosity)
-
Non-pyrogenic mannitol: 127.5 mg (stabilizing and isotonic agent)
technical information
-
form: lyophilized powder to be reconstituted with sterile water for injection (WFI)
-
application site: deep dermal layer or subcutaneous tissue
-
injection parameters: 25G or 26G needle; fan, retrograde, or depot technique
-
treatment cycle: typically 2-3 sessions; assessment of results no earlier than 4 weeks post-injection due to the gradual process of collagen biosynthesis
-
volume: 2 vials (150 mg PLLA each)
contraindications
-
history of hypersensitivity to PLLA, lidocaine (if added), or excipients
-
history of severe allergic and anaphylactic reactions
-
active inflammatory conditions (infections, cysts, acne, rashes) at or near the treatment area
-
tendency for keloid formation and connective tissue hypertrophy
|
VIAL |
||
|
The product may only be used by professionals. By making a purchase, you declare that you are a doctor or cosmetologist trained in aesthetic medicine. |
||