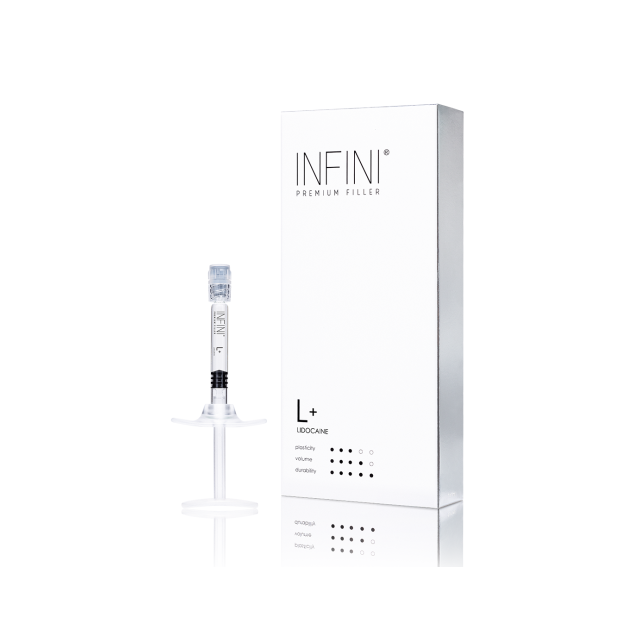
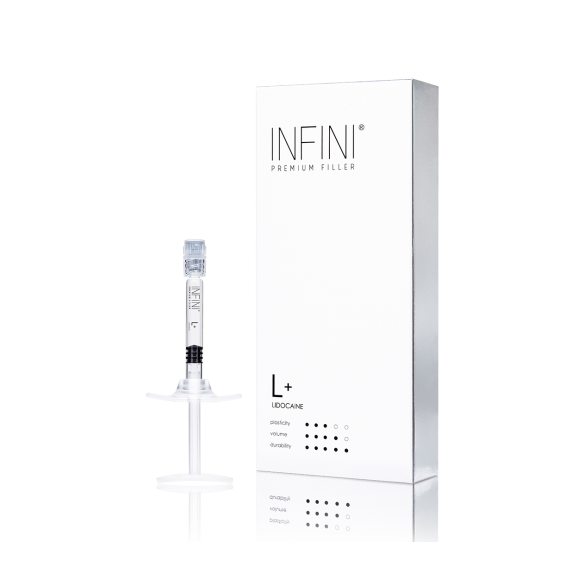
INFINI Premium Filler L+ Lidocaine (1x1ml) :: EXPIRY 02/2026
The lowest price of the product zł169.00 in day 31.01.2026
Lowest price in the last 30 days: zł169.00
Short expiration date - 16.02.2026
INFINI Premium Filler L+ Lidocaine is a sterile, monophasic, viscoelastic hydrogel implant based on highly cross-linked hyaluronic acid of non-animal origin. Utilizing Hy-BRID cross-linking technology and large-sized particles (600 μm), the preparation is characterized by high density and significant tissue lifting capacity. The device exhibits high mechanical stability at the injection site while maintaining optimal cohesivity. The formula contains lidocaine hydrochloride as an ancillary substance with a local anesthetic effect, improving patient tolerance during the procedure.
indications and intended use
The device is intended for administration via injection by authorized medical personnel to:
-
facial volumetry and contouring: provide physical restoration of soft tissue volume in areas affected by atrophy
-
correction of deep structural defects: provide mechanical filling of deep wrinkles, nasolabial folds, and marionette lines
-
modeling of anatomical structures: provide structural correction of the nasal bridge and improvement of chin projection and shape
-
intensive lip modeling: provide volumetric correction of the vermillion border requiring significant lifting and definition
mechanism of action
Sodium hyaluronate at a concentration of 24 mg/ml serves as a space-occupying implant. Due to the large particle size and high degree of cross-linking, the gel creates a stable subcutaneous scaffold resistant to compression forces. The purification process in Hy-BRID technology minimizes residual cross-linking agent (BDDE) content, reducing the risk of immunological tissue reactions. The estimated duration of the mechanical effect is up to 10 months.
injection technique
-
depth: deep layers of the dermis or subcutaneous tissue (depending on the treatment area)
-
technique: linear-retrograde, fanning, bolus (depot), or cross-hatching technique
composition
-
Cross-linked Sodium Hyaluronate: 24 mg/ml (particle size 600 μm)
-
Lidocaine Hydrochloride: 3 mg/ml (0.3%)
-
Phosphate buffer pH 7.0 q.s
contents
-
1 sterile pre-filled syringe (1 ml)
-
2 sterile 27G x 13 mm needles
|
PRE-FILLED SYRINGE |
||
|
The product may only be used by professionals. By making a purchase, you declare that you are a doctor or cosmetologist trained in aesthetic medicine. |
||