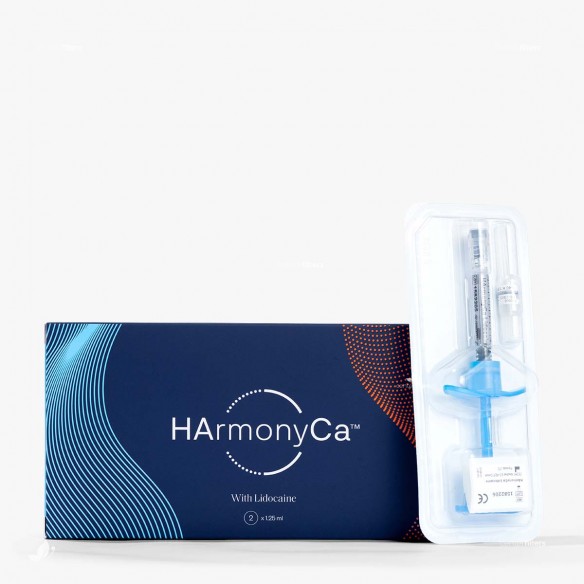
HarmonyCa with Lidocaine (1x1.25ml)
The lowest price of the product zł739.00 in day 01.02.2026
Lowest price in the last 30 days: zł739.00
HArmonyCa™ is a sterile, non-pyrogenic, hybrid Class III medical device, combining two active components: calcium hydroxylapatite (CaHA) microspheres and cross-linked hyaluronic acid (HA). The preparation is intended for injection to correct deep tissue deficits, restore volume, and provide biomechanical skin stimulation. The lidocaine content provides local anesthetic action, improving the tolerance profile of the injection procedure.
mechanism of action
-
immediate volumetric correction: cross-linked hyaluronic acid at a concentration of 20 mg/ml acts as a physical filler that lifts tissues, reduces wrinkles, and restores volume deficits immediately upon administration
-
long-term induction of neocollagenesis: calcium hydroxylapatite microspheres (55.7%) create a scaffold within the ecm matrix, which induces mechanical stimulation of fibroblasts, leading to the production of endogenous collagen and lasting skin restructuring
-
optimization of tissue stability: the hybrid structure provides a synergistic effect – ha delivers immediate hydration and lift, while caha guarantees long-term reinforcement of tissue density and cohesion
-
alleviation of discomfort: lidocaine hydrochloride inhibits nerve conduction at the injection site, minimizing the patient's sensory trauma during the implantation of the preparation
indications and intended use
The device is intended for administration via injection into the deep layers of the dermis by authorized medical personnel for:
-
volumetry and contour restoration: modeling of the facial oval, cheekbones, chin, and brow area
-
correction of deep skin folds: reduction of nasolabial folds, marionette lines, and "crow's feet"
-
non-surgical shape correction: modification of the profile and bridge of the nose
-
hand restructuring: improvement of soft tissue density on the dorsal surface of the hands
application areas
-
face (full spectrum of volumetry)
-
hands
composition
-
Calcium Hydroxylapatite (CaHA): 55.7% (microspheres sized 25-45 microns)
-
Cross-linked Hyaluronic Acid: 20 mg/ml (non-animal origin)
-
Lidocaine Hydrochloride: 3 mg/ml (0.3%)
-
Buffering system: physiological carrier
technical information
-
form: pre-filled syringe with a homogeneous hybrid suspension
-
application site: deep dermal layer or subcutaneous tissue
-
injection parameters: 27G x 13 mm needle (included in the set)
-
duration of effect: immediate mechanical effect transitioning into biological stimulation; clinical results may last from 12 to 36 months
-
volume: 1.25 ml
-
package contents: 1 pre-filled syringe, 1 needle (27G x 13 mm)
|
PRE-FILLED SYRINGE |
||
|
The product may only be used by professionals. By making a purchase, you declare that you are a doctor or cosmetologist trained in aesthetic medicine. |
||