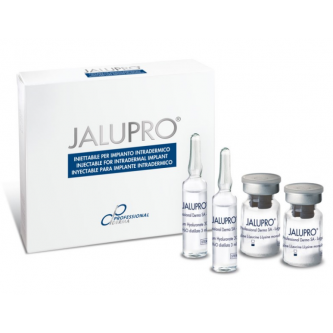
DIVES MED Hydra Royal (1x1ml)
The lowest price of the product zł109.00 in day 31.01.2026
Lowest price in the last 30 days: zł109.00
DIVES MED Hydra Royal is a sterile, biocompatible Class III medical device (CE0297, ISO 13485 certified) in the form of a viscoelastic solution of non-cross-linked hyaluronic acid (20 mg/ml). The preparation is an advanced hydro-biostimulator designed for multi-level revitalization of the dermis and epidermis. Due to its optimal molecular weight and high HA concentration, the device restores physiological water homeostasis, stimulates the remodeling of the extracellular matrix (ECM), and provides immediate improvement in the tissue's biomechanical parameters.
Mechanism of Action
-
endogenous hyaluronic acid reintegration: the delivery of high-concentration ha (20 mg/ml) replenishes deficits resulting from aging processes, restoring skin volume lost due to dehydration atrophy
-
neocollagenesis and elastogenesis stimulation: the presence of the preparation in the dermis induces mechanical stretching of fibroblasts, which activates the synthesis of collagen and elastin fibers, increasing tissue density and cohesion
-
hydro-dynamic micro-relief correction: the preparation exhibits a high capacity for binding water molecules, resulting in the mechanical lifting and smoothing of fine expression lines and dehydration lines
-
epidermal barrier revitalization: the multi-level action of the device optimizes keratinocyte metabolism, improving the structure and luminance of the superficial skin layers
-
oxidative stress protection: hyaluronic acid acts as a scavenger of free radicals, limiting the effects of photoaging and protecting the skin's protein structures from degradation
Indications and Intended Use
The device is intended for implantation by authorized medical personnel to:
-
intensive biorevitalization: restoring hydration, tension, and elasticity to the face and sensitive areas
-
aging defect correction: reduction of expression lines, including wrinkles in the periorbital area (around the eyes)
-
photoaging therapy: regeneration of skin with signs of solar degradation
-
vermilion border revitalization: nourishment, hydration, and improvement of the tension of the lip tissue
-
tissue volume replenishment: improving turgor and filling fine depressions caused by dehydration atrophy
Application Areas
-
face
-
eye area
-
lips
-
neck and décolletage
Composition
-
hyaluronic acid (non-cross-linked): 20 mg/ml
-
variant: lidocaine-free
Technical Information and Protocol
-
form: sterile pre-filled syringe with a capacity of 1 ml
-
application site: subcutaneous layer or deep layer of the dermis
-
needles included: 2 x 30G x 13 mm
-
clinical effect durability: approximately 6 months (depending on patient predisposition and injection technique)
-
quality standards: product certified in accordance with iso 13485 and directives for class iii medical devices
-
package contents: 1 x 1 ml pre-filled syringe (also available in multipacks of 3 x 1 ml)
|
MEDICAL DEVICE CE0297 |
||
|
STERILE |
||
|
PRE-FILLED SYRINGE |
||
|
Product to be used only by doctors or cosmetologists trained in aesthetic medicine. |
||