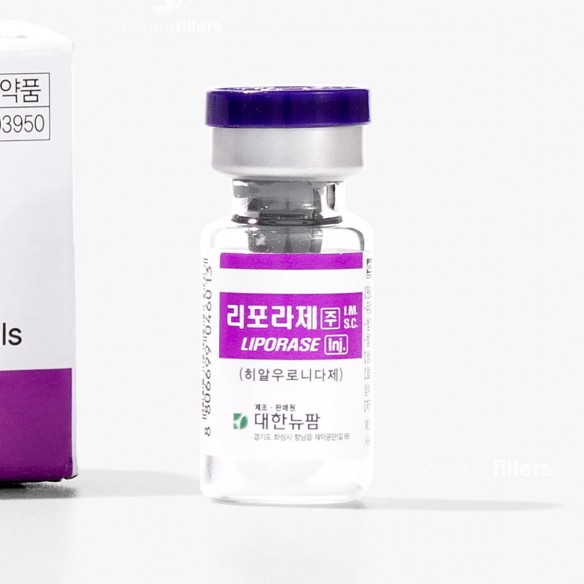
LIPORASE Hyaluronidase 1500 IU
hyaluronidase enzyme
volume: 1500 I.U.
formula
Product in the form of a powder to be activated with 3 ml of saline solution, designed to remove hyaluronic acid over-injections.
features
Designed to level unwanted effects or complications arising from hyaluronic acid over-injection, or unevenness arising from improper injection. Hyaluronidase is used in emergency situations where the filler has been accidentally injected into an artery causing obstruction of blood supply.
variants available
- without lidocaine
contains
powder in a vial: 1500 I.U.
indications
- removes excessive amounts of administered hyaluronic acid
- removes lumps and bumps at the site of acid implantation
- removes the acid after accidental injection of the filler into an artery or venous vessel, which would result in embolism and necrosis
- removes the acid in cases of intolerance to hyaluronic acid or crosslinking agent
- removes the acid in the case of filler displacement
depth of administration
intradermally
|
VIAL |
||
|
STERILE |
||
|
Product to be used only by physicians or cosmetologists trained in in the field of aesthetic medicine. |
||